Calculating Free Energies of Polymers Using ERmod and Designing Functional Polymer Membranes
Toray has launched its “Green Innovation Project,” which aims to contribute to addressing the Earth’s environmental issues through advanced materials, as a company-wide initiative. In this project, there are high expectations that polymer membranes will play indispensable roles in mitigating and addressing the Earth’s environmental problems. Examples include polymer electrolyte membranes used with fuel cells, which represent the next-generation energy source, and reverse osmosis membranes used for sea water desalination, which is a promising technology that will help secure water resources in arid regions.
To design polymer membranes with advanced selective permeability, we employ an approach called the solution diffusion model (see Figure). By calculating the diffusion coefficient for the diffusion term using molecular dynamics and by calculating the solvation free energy for the solution terms, a path to designing highly functional membranes through molecular simulations is opened. However, free energy is a physical quantity that requires extremely high computational load to calculate, and we needed a way to calculate the solvation free energy of small molecules into polymers using reasonable time and computational resources.
We have therefore commenced a research to apply ERmod, a high-speed and high-precision free energy model, to polymers. After investigating various techniques, including ways to calculate the interaction energy between small molecules and polymers, and improving the free energy functional, we have constructed a technique that can calculate the solvation free energy within a realistic time and precision [1]. Results obtained by applying this technique to high polymer electrolytes and separation membranes are starting to appear, and it is highly regarded within our corporation.
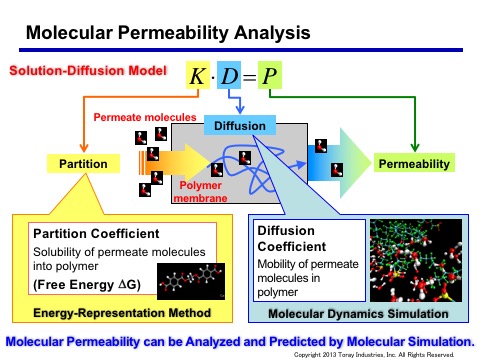
Figure: Conceptual diagram of the solution diffusion model. As it allows calculation of the solvation free energy with high speed and precision, the technique has become a practical tool.
[1] T. Kawakami, I. Shigemoto, and N. Matubayasi, J. Chem. Phys. 137, 234903 (9 pages) (2012).

